Targeted radioantibodies:
Striking with exceptional power
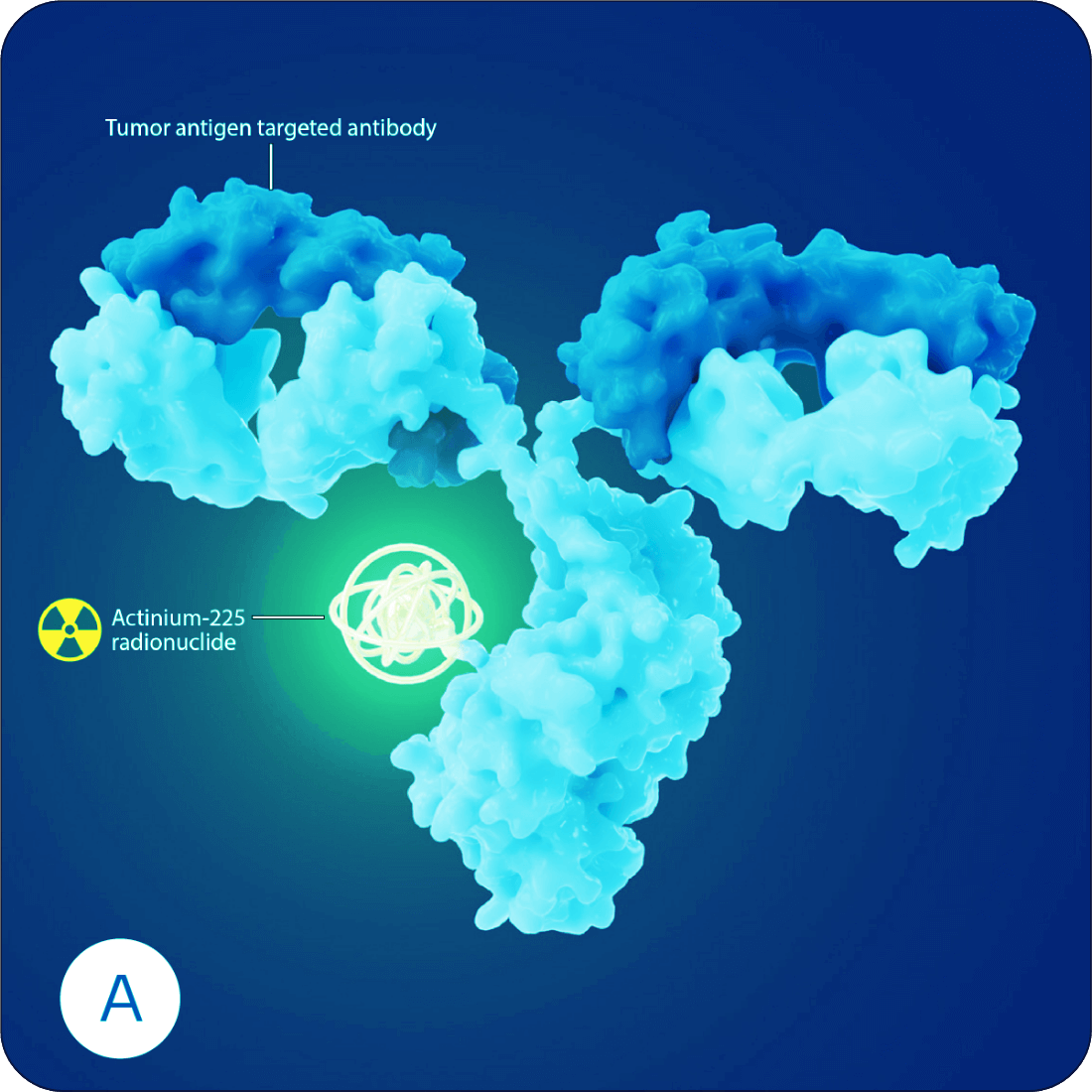
Radioantibodies are a type of targeted therapy where an antibody is linked to a radioactive payload. This radioactive payload holds the power to kill cancer cells by destroying the cell from within.1
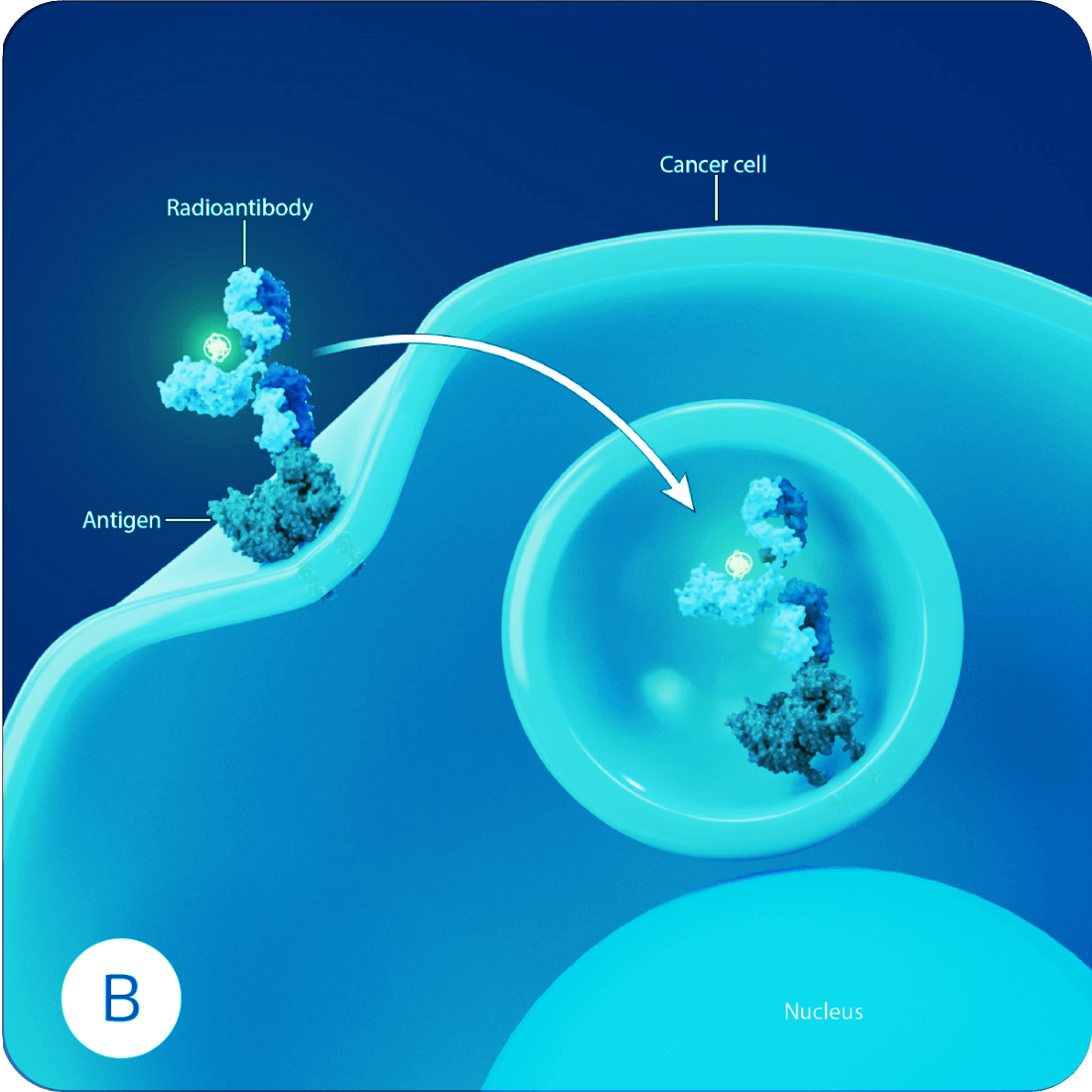
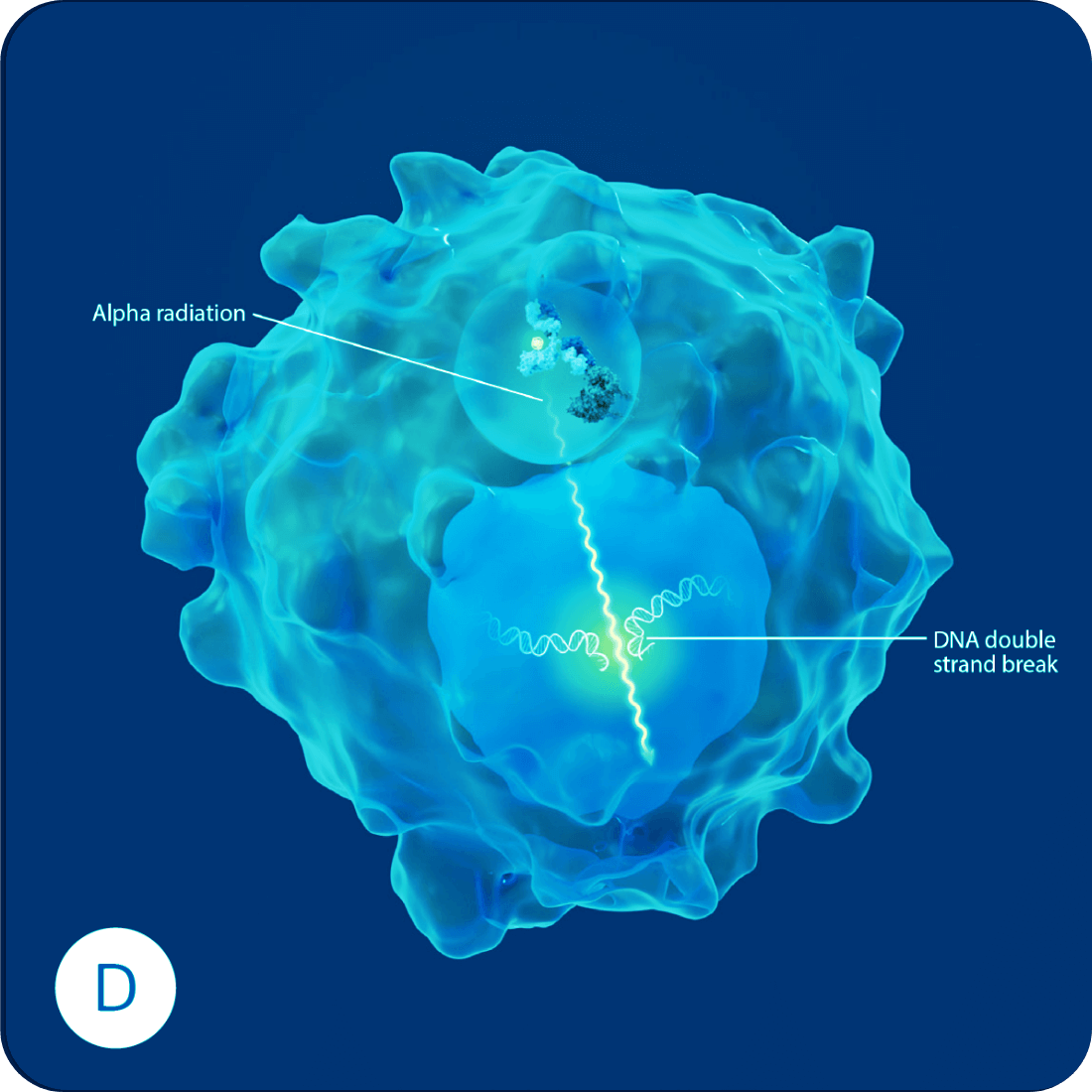
Each radioantibody is created by carefully selecting an antibody with a high affinity for a specific cancer target. This enables the radioantibody to home in and lock onto the surface of a cancer cell. Once attached, the radioantibody is drawn into the cells, delivering the radioactive payload adjacent to the nucleus. The resulting radiation field causes irreparable damage to the DNA, eventually killing the cancer cell. And because each radioantibody is carefully tailored to specifically target the cancer cells and the alpha radiation has a more focused range of emission, damage to healthy tissue is minimized.
Our technology improves upon the limitations of current radiotherapies, and we’re already making progress. Our research has determined that alpha-emitting radioantibodies offer improved precision and potency over other technologies, including those relying on beta-emitting isotopes such as lutetium-177 (Lu-177).




Unleashing the
power of
radioantibodies
Convergent is developing radioantibodies that seek to improve
upon the safety and efficacy issues of current radiotherapies.
1. Mulford DA, Scheinberg DA, Jurcic JG. The promise of targeted {alpha}-particle therapy. J Nucl Med. 2005;46(Suppl 1):199S-204S.