Prostate cancer
CONV01-α: An alpha-emitting radioantibody with a promising clinical future
CONV01-α (formerly Ac-225-J591) is a proprietary, best-in-class radioantibody targeting a protein (antigen) expressed on the surface of almost all prostate cancer cells.
CONV01-α combines the precision and pharmacokinetics of antibodies with the tumor-killing potential of alpha-emitting radionuclides (a radioactive isotope that delivers the radiation inside cancer cells).
Selection of an alpha radionuclide is significant. The promise of their potency comes from the high destructive energy they emit with the short range that energy travels. Once inside a cell, these characteristics combine for greater potential for tumor cell destruction. In contrast to the longer range of beta emitters, the short range of alphas is less likely to damage healthy adjacent cells and tissues.3
In CONV01-α, the antibody is linked to a powerful alpha radionuclide called Actinium-225 (Ac-225) which has a half-life of 10 days—ideal for production and distribution.4
The advantages of alpha-emitting payloads vs beta-emitting payloads
Each particle emits energy, but with very different properties.
| Alpha (α) particle | Beta (β) particle | |
|---|---|---|
| Size of the particle | 7300x bigger than a beta particle | One of the smallest particles |
| How far the particle travels | Usually 3-5 cell diameters, minimizing damage to surrounding healthy tissues | Travels far beyond the cancer cell, likely causing damage to healthy tissue |
| Energy released | 100 keV of energy | 0.2 keV of energy |
| Type of DNA breakage (damage) | Double strand | Single strand |
| How many particles needed to kill a single cancer cell | 1 | 1000 |
Choosing an alpha radionuclide was a critical choice—and pairing it with a highly selective antibody is imperative to create a therapy that can effectively treat cancer.
Monoclonal antibodies have a greater affinity for targets such as prostate-specific membrane antigen (PSMA), a type of protein found on the surface of prostate cancer cells. Monoclonal antibodies also offer improved tumor internalization and retention to allow for greater potency, fewer doses, and less overall radiation administered compared with beta- or alpha-small molecule ligands.
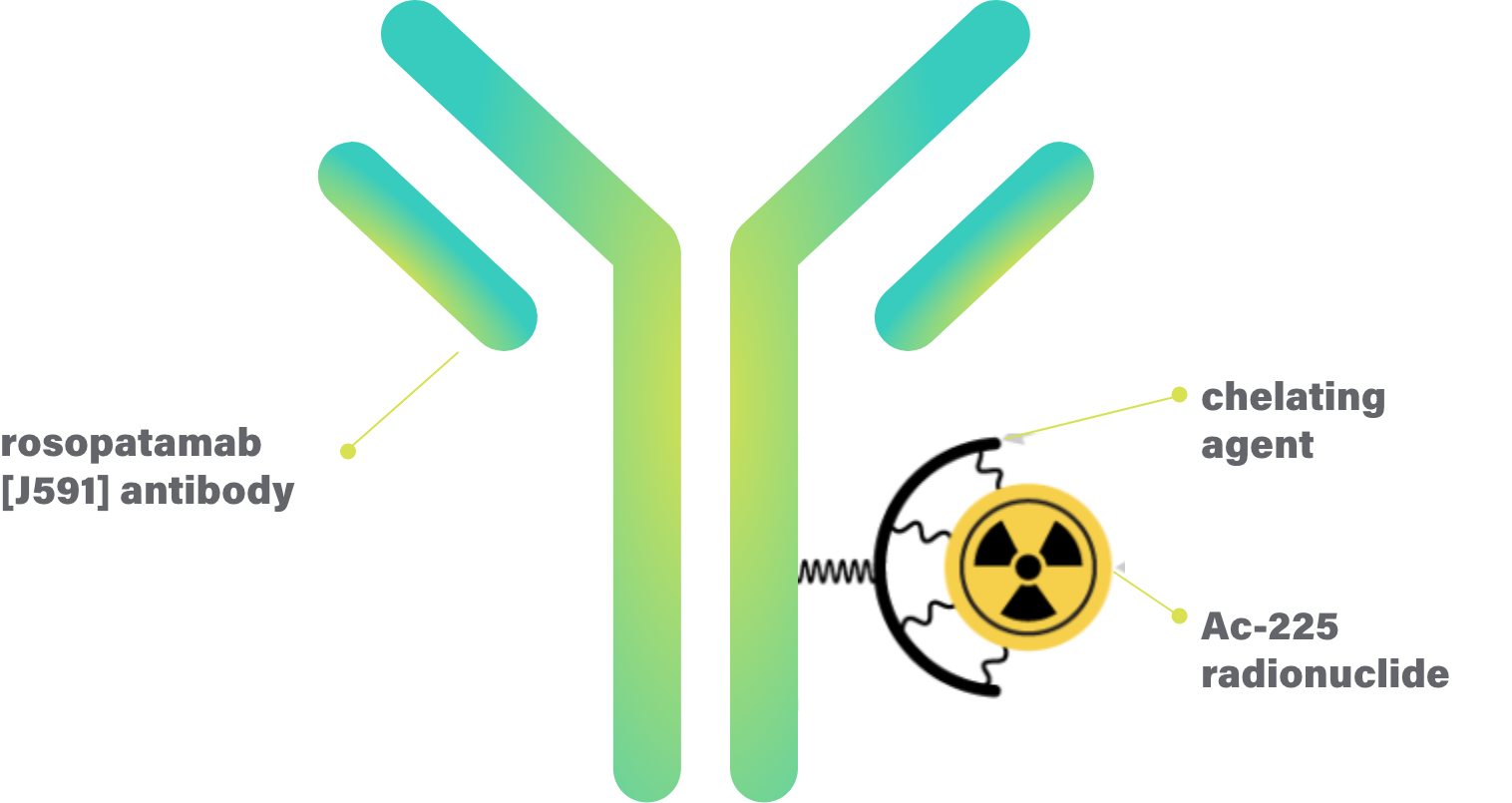

CONV01-α: Designed to precisely deliver potent, tumor-killing alpha radiation to malignant cells while sparing healthy tissue because of favorable antibody pharmacokinetics and the superior qualities of alpha emitters.
By linking Actinium-225 to a tumor-targeting antibody, the delivery of tumor-killing radiation is increased while greatly reducing both off-tumor effects and the amount of radiation delivered per treatment course, improving both treatment efficacy and safety.
Clinical trials are ongoing; early results are positive.
CONV01-α: Proof of concept in early clinical trials
Results from early clinical trials demonstrated that CONV01-α decreases blood levels of prostate-specific antigen (PSA), a biomarker used to evaluate prostate cancer disease burden and progression.
A robust clinical
development plan
Our approach to radiopharmaceutical development has led to a promising candidate, CONV01-α, which has already produced encouraging clinical data.
Recruitment for CONVERGE-01 is underway and planning for future trials continues.
Compassionate use statement
Convergent Therapeutics, Inc. (“Convergent”) is a biotechnology company focused on developing next-generation targeted radiotherapies to treat cancer. At this time, Convergent does not provide access to investigational products outside of clinical trials. We encourage patients to participate in clinical trials of our investigational product whenever possible. Clinical trials are designed, conducted, and monitored to ensure that the safety and effectiveness of investigational products are appropriately evaluated before the results from the trials are submitted to regulatory agencies for review with the intent to make them more broadly available to patients. You and your health care provider may learn more about our clinical trial visiting www.clinicaltrials.gov and searching for Convergent Therapeutics. If you are a health care provider who is interested in learning more about one of our investigational products, or a physician with questions about participation in one of our clinical trials, please submit a request to info@convergentrx.com.
1. Cancer Facts and Figures 2024. American Cancer Society. Accessed December 12, 2024. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2024/2024-cancer-facts-and-figures-acs.pdf 2. Kirby M, Hirst C, Crawford ED. Characterising the castration-resistant prostate cancer population: a systematic review. Int J Clin Pract. 2011;65(11):1180-1192. doi:10.1111/j.1742-1241.2011.02799.x 3. Mulford DA, Scheinberg DA, Jurcic JG. The promise of targeted {alpha}-particle therapy. J Nucl Med. 2005;46(Suppl 1):199S-204S. 4. The Goldilocks of radioisotopes, just right for treating cancer. Brookhaven National Laboratory. Published August 2, 2022. Accessed September 20, 2024. https://bnl.gov/newsroom/news.php?a=220754#~:text=Because%20of%20the20types%20of,for%20fighting%20some%20tough%20caners 5. Tagawa ST, Thomas C, Sartor AO, et al. Prostate-specific membrane antigen-targeting alpha emitter via antibody delivery for metastatic castration-resistant prostate cancer: a phase I dose-escalation study of 225Ac-J591. J Clin Oncol. 2024;42(7):842-851. doi:10.1200/JCO.23.00573 6. Nauseef JT, Sun M, Thomas C, et al. Abstract CT014: Phase I dose-escalation study of fractionated dose 225Ac J591 for metastatic castration resistant prostate cancer. Cancer Res. 2023;83(Suppl 8). doi:10.1158/1538-7445.AM2023-CT014